Macromolecules & The Chemistry of Life
At the heart of every cell, snack, seed, and strand of DNA are carbon-based molecules playing molecular Tetris.
The Dive
Carbon is the superstar of organic chemistry. It can form four bonds, meaning it’s basically the social butterfly of atoms—always ready to link arms and build something new. That’s why it’s the backbone of all life’s molecules (except water, which gets an honorable mention).
Macromolecules are big, complex molecules made by linking smaller building blocks called monomers. When you snap monomers together like molecular LEGO bricks, you get a polymer—or what we call a macromolecule.
There are four major classes of macromolecules essential to life: carbohydrates, lipids, proteins, and nucleic acids. Each has a different job, a different shape, and a different story to tell.
Carbohydrates are your body's quick and steady energy source. Monosaccharides (like glucose) are simple sugars, while complex carbs like starch and cellulose provide slow-release fuel and structural support in plants.
Lipids—aka fats and oils—are long-term energy storage molecules. Built from glycerol and fatty acids, they include saturated fats (from animals) and unsaturated fats (from plants). Then there are trans fats: artificially altered oils that were great for shelf life but bad for heart health.
Proteins do it all. They’re made of amino acids and are the Swiss Army knives of biology—enzymes, hormones, structural components, and more. Each protein’s shape determines its job. Remember: form follows function.
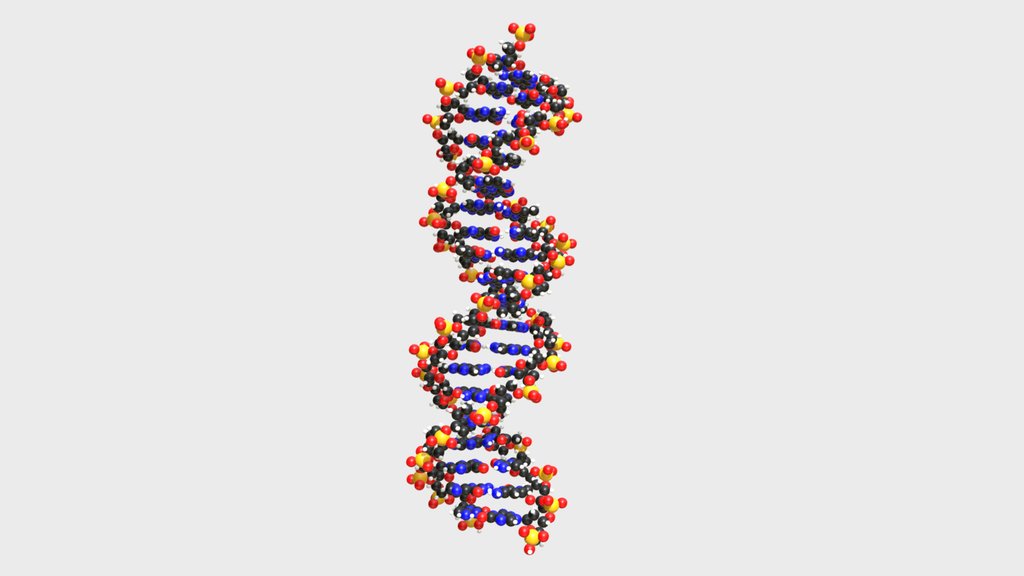
Nucleic acids (DNA and RNA) are your body’s data storage and instruction manuals. Made from nucleotides, they hold the genetic code for building every protein in your body. Without them, your cells wouldn’t know what to do next.
Why It Matters
Every bite you take, breath you draw, and cell that divides depends on macromolecules. They’re not just the building blocks of life—they’re its architects, messengers, energy sources, and information keepers. Understanding them is like reading life’s instruction manual, molecule by molecule.
?
Why can carbon form so many different molecules compared to other elements?
How do the structures of carbohydrates and lipids influence how they’re used in your body?
What happens when proteins are misfolded or damaged?
How do mutations in DNA affect the proteins your body makes?
Why did scientists originally think trans fats were healthy, and what changed?
Dig Deeper
Despite the diverse appearance and characteristics of organisms on Earth, the chemicals that make up living things are remarkably similar, often identical. In this episode of Crash Course Biology, we’ll look at the building blocks of the four major classes of biomolecules, how those join up to form macromolecules, and how a team of six atoms forms the vast majority of living matter.
A quick and engaging breakdown of the four major macromolecules and how they fuel and structure life. Ideal for anyone looking to connect biology and chemistry.
Related

DNA: The Letters That Define Life
DNA is the biological code that tells every living cell how to grow, function, and pass life forward.

The Properties of Water
Water isn’t just wet. It’s weird—and wonderful. From defying gravity in trees to sticking to itself in your bloodstream, water’s molecular magic is what makes life on Earth possible.

How Microplastics Are Shaping Our Health and Environment
Microplastics are everywhere—from the food we eat to the air we breathe. But what are the hidden costs of these tiny pollutants, and how can we stop them?
Further Reading
Stay curious!